Abstract
Background/introduction: The Bcl-2 inhibitor venetoclax has exhibited activity in pts with NHL in monotherapy (Clin Cancer Res 2021;27:4690-5) and in combination regimens (Blood 2019;133;1964-7). BGB-11417 is a highly selective Bcl-2 inhibitor with potency >10 times that of venetoclax in biochemical assays. BGB-11417 has favorable pharmacokinetics and a broad therapeutic index that may result in an improved safety profile. BGB-11417 monotherapy is tolerable, with no maximum tolerated dose (MTD) reached after dose escalation through all planned doses to 640 mg once daily (QD) in pts with NHL (EHA 2022. Abstract P687).
The combination of Bcl-2 and Bruton tyrosine kinase (BTK) inhibitors is tolerable with synergistic activity in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and mantle cell lymphoma (MCL) (J Clin Oncol 2019;37:2722-9; N Engl J Med 2019; 380:2095-103; ASH 2020 Abstract S158; N Engl J Med 2018;378:1211-23). ZANU is a next-generation BTK inhibitor with favorable activity and safety profiles in pts with CLL/SLL (EHA 2021 Abstract LB1900) and WM (Blood. 2020;136(18):2038-2050) with FDA approval for treatment in MCL, marginal zone lymphoma (MZL), and WM. BGB-11417-101 is an ongoing first-in-human phase 1/1b dose-escalation/expansion study (NCT04277637). Data from separate cohorts of MCL, WM and combined NHL (Follicular lymphoma [FL], diffuse large B cell lymphoma [DLBCL], MZL, transformed FL) are presented here.
Methods: In the monotherapy cohorts, pts received BGB-11417 (40, 80, 160, 320, or 640 mg QD) with a ramp-up to the intended dose. In combination cohorts, pts received ZANU (320 mg QD or 160 mg twice daily) 8-12 weeks before BGB-11417. Dose-limiting toxicity for each dose cohort was evaluated by a Bayesian logistic regression model during dose ramp-up through day 21 at the intended dose. Responses were assessed per Lugano criteria. Adverse events (AEs) were reported per Common Terminology Criteria for AEs v5.0, and tumor lysis syndrome (TLS) was assessed per Howard (2011) criteria.
Results: As of May 15 2022, 45 pts with NHL, WM, or MCL received BGB-11417 (34 monotherapy; 11 combination). Monotherapy pts (n=28 NHL [n=18 DLBCL, n=6 FL, n=4 MZL]; n=6 WM) received BGB-11417 doses ≤640 mg. Combination pts (n=11 MCL) received ZANU, and 9 (82%) had also received BGB-11417 doses ≤160 mg (data include 2 pts who are still in the pretreatment phase with ZANU). Dose escalation to 640 mg was completed for NHL monotherapy; all planned doses were tested, with no MTD reached. Dose escalation is ongoing for monotherapy in WM and combination therapy in MCL cohorts. Median follow-up was 6.5 months (range 0.4-25.3) for monotherapy and 4.8 months (range 0.4-8.9) for combination therapy.
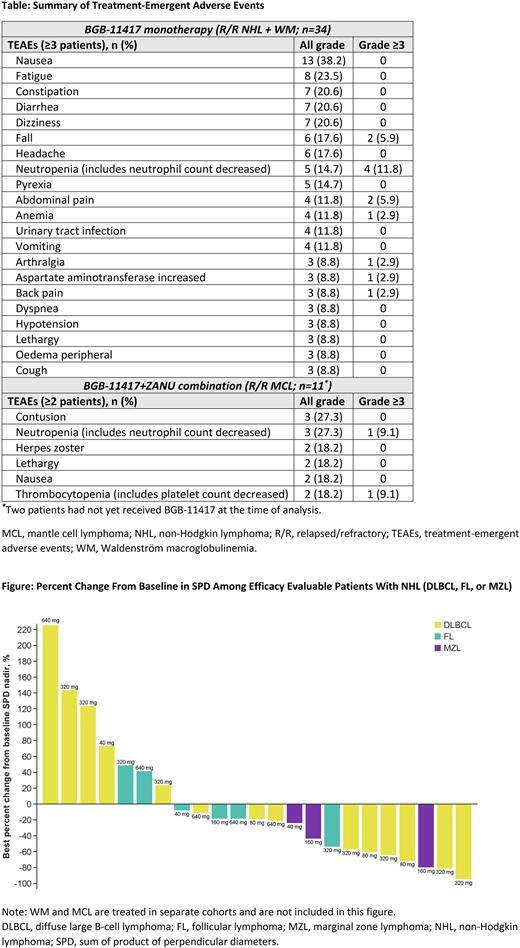
Treatment-emergent AEs (TEAEs) across all dose levels are listed in the Table. For monotherapy, the most common TEAEs (≥20%) were nausea (38%), fatigue (24%), constipation, diarrhea and dizziness (21% each); the most common grade ≥3 TEAE was neutropenia (12%). For combination therapy, the most common TEAEs were contusion and neutropenia; grade ≥3 AEs were infrequent. Twenty-five monotherapy pts (22 disease progression [PD]; 1 AE; 2 other reasons) and 2 combination therapy pts (PD) discontinued treatment. No TEAEs leading to death and no TLS were reported to date.
Amongst combined NHL cohorts, 23 pts reached the first response assessment time point, but most were treated below the recommended phase 2 dose (RP2D). Of these pts, 3 responded (n=2 DLBCL, n=1 MZL) including 1 complete response (DLBCL), and notable reductions in the sum of the product of perpendicular diameters (SPD) were seen (Figure). In the MCL combination cohort, 6 of 11 (55%) pts responded. In the monotherapy WM cohort, 1 of 4 evaluable pts exhibited minor response at the first dose level tested (80 mg); hemoglobin count increases of more than 20 g/L were seen in 3 of 6 treated pts and all remain on treatment.
Conclusion: These initial data show an encouraging safety profile and preliminary evidence of efficacy for BGB-11417 in NHL, MCL, and WM cohorts. No MTD was reached even at the highest dose level of 640 mg QD. All low-grade TEAEs and grade ≥3 neutropenia were manageable. The response data includes NHL patients mostly treated at doses below the RP2D; longer follow-up of BGB-11417 monotherapy and combination therapy at the RP2D is needed. Monotherapy MCL data are forthcoming.
Disclosures
Soumerai:GlaxoSmithKline: Research Funding; Moderna: Research Funding; TG Therapeutics: Consultancy, Research Funding; Abbvie: Consultancy; AstraZeneca: Consultancy; Bristol Myers Squibb: Consultancy; Verastem: Consultancy; Biogen: Consultancy; Roche: Consultancy; MEI: Research Funding; Adaptive Biotechnologies: Research Funding; Genentech/Roche: Consultancy, Research Funding; Boston Gene: Research Funding; Beigene: Consultancy, Research Funding. Lasica:Janssen: Other: Education. Opat:AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Antengene: Consultancy; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Consultancy; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding. Cheah:Roche: Consultancy, Honoraria, Other: Travel expenses, Research Funding; Janssen: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Ascentage Pharma: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; BeiGene: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding. Chan:GSK: Membership on an entity's Board of Directors or advisory committees; Eusa: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Verner:Janssen Cilag Pty Ltd: Research Funding. González Barca:Janssen: Honoraria, Other: Travel, Accommodations, Expenses; AbbVie: Honoraria, Other: Travel, Accommodations, Expenses; Takeda: Honoraria; EUSA Pharma: Honoraria; AstraZeneca: Honoraria; Roche: Other: Travel, Accommodations, Expenses. Tedeschi:AstraZeneca: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Beigene: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau. Hilger:BeiGene: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months. Simpson:BeiGene: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Other: Travel, Accommodations, Expenses. Tam:LOXO: Honoraria; AstraZeneca: Honoraria; Beigene: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Janssen: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal